× nearby
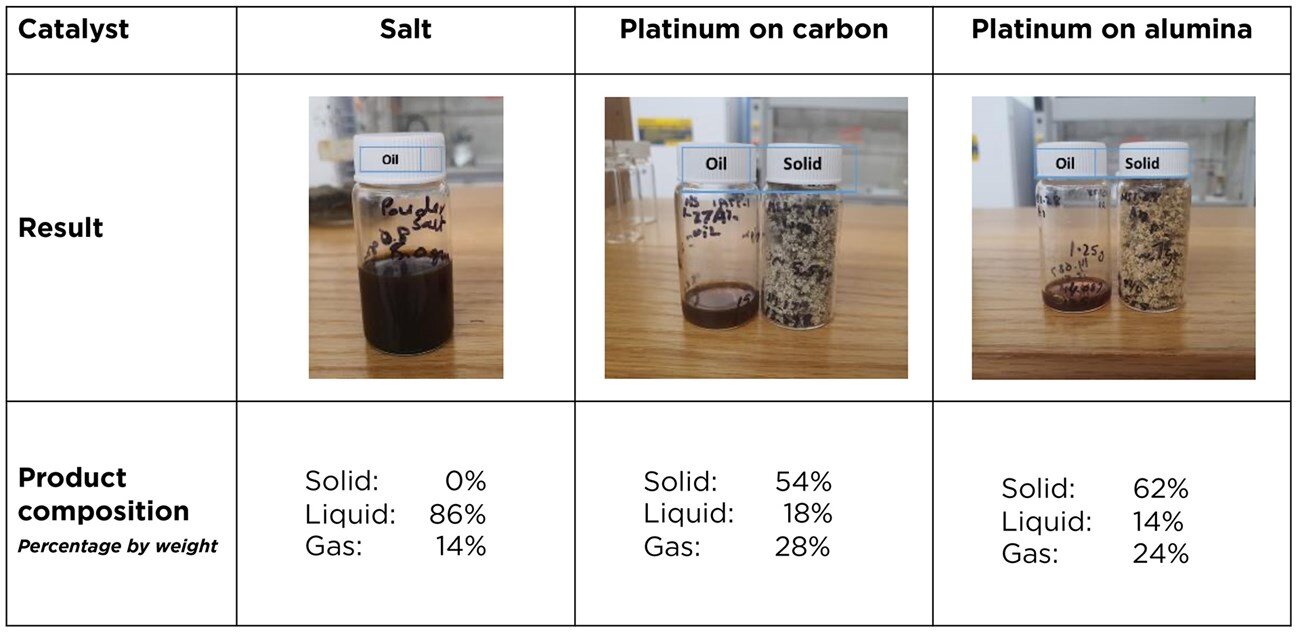
In an experiment carried out at Michigan State University, the pyrolysis of polyolefins using table salt as a catalyst prevented the unwanted solid byproduct, which is produced by catalysts based on expensive platinum. Credit: Shaker, M. et al., Advanced Materials2023
Muhammad Rabnawaz, an associate professor at Michigan State University’s School of Packaging and an inductee into the National Academy of Inventors, always believes that the smartest solution is the simplest.
That belief is reflected in his team’s new journal publication Advanced Sustainable Systems.
Rabnawaz and his colleagues show that sodium chloride—table salt—can outperform more expensive materials being studied to help recycle plastics.
“This is really exciting,” said Rabnawaz. “We need simple, low-cost solutions to tackle a big problem like plastic recycling.”
Although plastics have historically been marketed as recyclables, the reality is that nearly 90% of plastic waste in the United States ends up in landfills, incinerators or as environmental pollution.
One of the reasons why plastics are thrown away so much is that the materials obtained from recycling are not valuable enough to spend the money and resources required to obtain them.
According to the group’s estimates, table salt can transform the economy and significantly reduce costs when it comes to the process of regeneration known as pyrolysis, which works by combining heat and chemistry.
Although Rabnawaz expected salt to have an effect because of how well it conducts heat, he was still surprised by how well it worked. It’s passed on expensive materials—chemicals designed to catalyze the reaction together—and he believes his team is only just beginning to harness its potential.
Furthermore, the work is already getting attention from big names in the industry, he said.
In fact, the research was partially funded by Conagra Brands, a consumer packaged goods company.
A catalyst worth its salt
Pyrolysis is a process that breaks down plastics into a mixture of simple, carbon-based substances, which come out in three forms: gas, liquid oil and solid wax.
This wax component is often undesirable, Rabnawaz said, but it can account for more than half of the products, by weight, of current pyrolysis methods. The same is true when using catalysts, which are useful, but often too toxic or too expensive to use in plastic waste management.
For example, Platinum has very attractive properties, which is why it is used in catalytic converters to reduce harmful emissions in cars. But it is also very expensive, which is why thieves steal catalytic converters.
Although pirates can’t steal platinum-based materials from a pyrolysis reactor, trying to recycle plastics and those materials would require a huge investment — millions, if not hundreds of millions, of dollars, Rabnawaz said. And current catalysts are not efficient enough to justify that price.
“No company in the world has this kind of money to burn,” Rabnawaz said.
In previous work, Rabnawaz and his team showed that copper oxide and table salt worked as catalysts to break down the plastic known as polystyrene. Now, they have shown that table salt alone can eliminate the wax byproduct from the pyrolysis of polyolefins – polymers that account for 60% of plastic waste.
“That first paper was important, but I didn’t get excited until we were working with polyolefins,” Rabnawaz said. “Polyolefins are great, and we’re still outpacing expensive catalysts.”
Joining Rabnawaz on the project were Christopher Saffron, associate professor in the College of Agriculture and Natural Resources, visiting scholar Mohamed Shaker and MSU doctoral student Vikash Kumar.
By using table salt as a medium to pyrolyze polyolefins, the team produced a liquid oil containing hydrocarbon molecules similar to those found in diesel fuel, Rabnawaz said. Another perk of the salt catalyst, the researchers point out, is that it can be reused.
“You can get the salt by washing the recovered oil with water,” said Rabnawaz.
Researchers have also shown that table salt aids in the pyrolysis of metallic plastic films, commonly used in food packaging, such as potato chip bags, which can be reused.
Although pure table salt did not succeed the platinum-alumina catalyst the team also tested with metalized films, the results are the same, and the salt is a fraction of the cost.
Rabnawaz, however, stressed that metallic films, while useful, are inherently problematic. He sees a world where such films are no longer needed, which is why his team is working to replace them with sustainable materials.
The group will continue to work to advance its pyrolysis project.
For example, the team has not fully identified the products of pyrolysis gas with table salt. And Rabnawaz believes that the team can improve this method so that liquid products contain chemicals with valuable applications rather than being burned as fuel.
However, the immediate return of new table salt tactics is encouraging. Based on preliminary economic analysis the team estimates that a commercial pyrolysis reactor could triple its profitability by adding salt.
More information:
Mohamed Shaker et al, Transforming Plastic Recycling Chemicals with Table Salt, Advanced Sustainable Systems (2023). DOI: 10.1002/adsu.202300306
#Table #salt #safe #inexpensive #reusable #obtain #products #plastic #waste