Researchers are gaining insight into how schizophrenia develops by studying the strongest known genetic risk factor. When a small part of chromosome 3 is missing, called 3q29 deletion syndrome, it increases the risk of schizophrenia about 40-fold.
By analyzing how the patterns of altered gene activity correspond in two models of 3q29 deletion syndrome – in mice and in organoids of the human brain – scientists have observed an unexpected change in brain cells: mitochondrial dysfunction.
The results coincide with work on another genetic risk factor for schizophrenia, the 22q11 deletion syndrome or DiGeorge syndrome, which has also been found to involve disruption of mitochondrial function.
The results are published in Science Advances.
“For the genetic changes associated with schizophrenia, we want to understand the underlying pathology at the cellular level,” said lead author Ryan Purcell, PhD, assistant professor of cell biology at Emory University School of Medicine. “This gives us an opportunity, which can help cut through the complexity of schizophrenia’s polygenic and better understand its neurobiology.”
The research resulted from a collaboration between researchers at Emory and Rutgers. At Emory, the collaboration included the laboratories of co-senior author Gary Bassell, PhD; Steven Sloan, PhD; and Victor Faundez MD, PhD. The lead author is Emory postdoctoral fellow Esra Sefik, PhD, now at Princeton.
Senior author Jennifer Mulle, PhD, and her colleagues first showed that the 3q29 deletion was a risk factor for schizophrenia in 2010. Previously at Emory, Mulle is an associate professor of psychology, neuroscience and cell biology at Rutgers.
“Our data provide strong support for the hypothesis that mitochondrial dysregulation contributes to the development of schizophrenia,” said Mulle. “The connection between mitochondrial dynamics and neuronal maturation is an important area for further detailed and rigorous research.”
3q29 deletion syndrome has an estimated prevalence of about 1 in 30,000. In addition to increasing the risk of schizophrenia, deletion of 3q29 can include mental retardation, autism spectrum disorder and congenital heart defects. The effect of 3q29 deletion on the risk of schizophrenia is greater than that of any other known genotype, but the contributions of specific genes among deletions are still being distinguished.
The discovery that the chromosomal deletions of schizophrenia are associated with mitochondria is contrary to the expectations of the field that such changes should change the proteins involved in synapses: communication between neurons. However, mitochondria are critical to the functioning of energy-starved synapses – so these models may not conflict. More work is needed to determine whether mitochondria in synapses are more vulnerable, Bassell said.
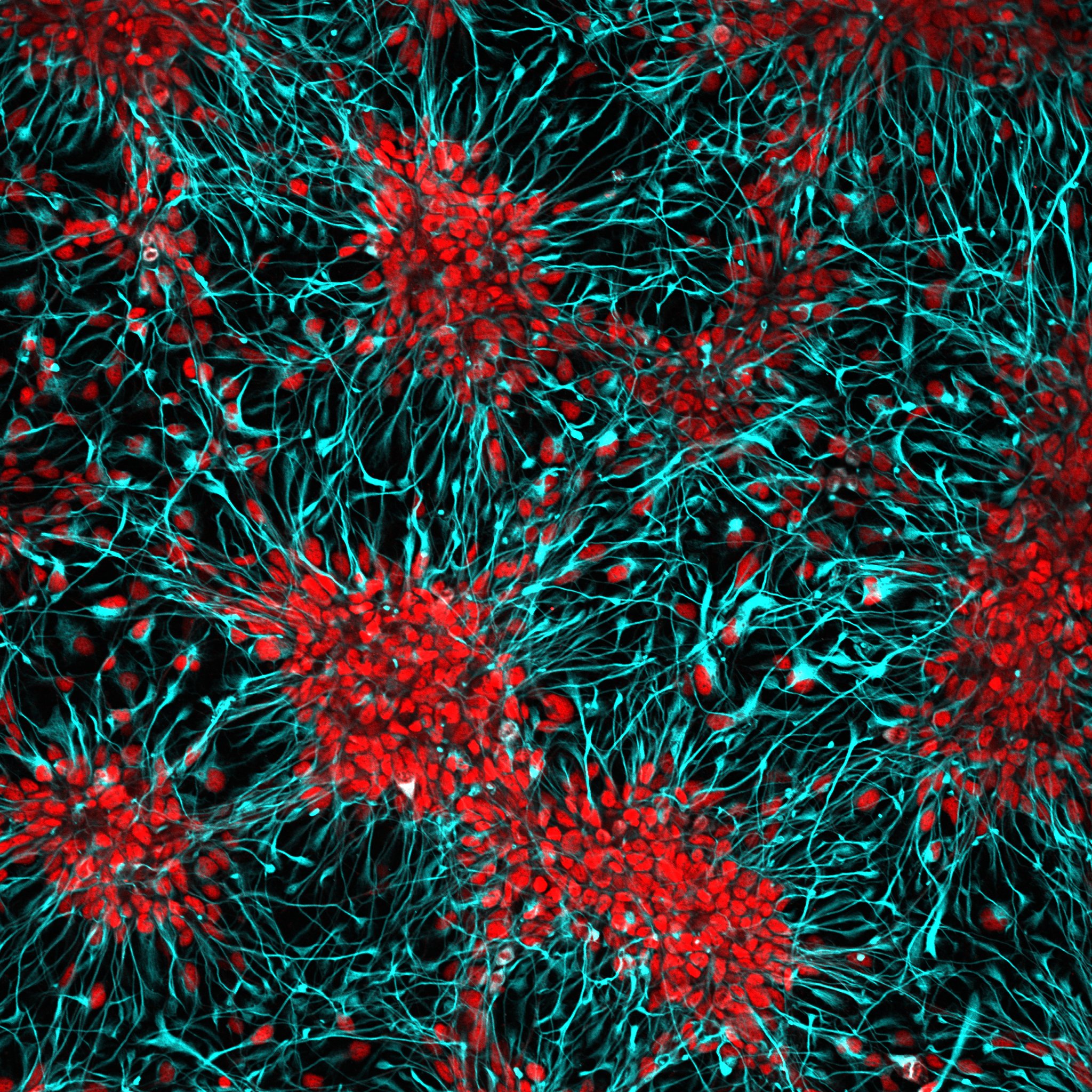
Finding that 3q29 cells have dysregulated mitochondria was also surprising because only one of the 22 genes in the deletion encodes a protein located in the mitochondria. In contrast, the deletion of 22q11 contains several types of mitochondrial protein encoding. The 3q29 gene involved may regulate the production or import of mitochondrial proteins, Purcell says.
Mitochondria are found in every cell – they consume oxygen to produce energy in the form of ATP (adenosine triphosphate). Mitochondria have their own genomes, but most of the proteins found within them are encoded by genes in the cell’s nucleus.
As a result of the modification of mitochondrial function, 3q29 cells do not have metabolic flexibility, which means that they have difficulty adapting to changes in energy sources. This can interfere with neuronal development, because growing neurons must change to rely more on mitochondria for energy as they divide.
Supported by recently awarded funding, Purcell is now testing whether cells from people with 22q11 also show similar metabolic changes.
“Ultimately, we want to understand which cellular changes like these are associated with specific clinical outcomes, which can help design more effective treatments,” Purcell said.
The results reported in Science show how the deletion of 3q29 affects the whole body, not just the brain; effects on mitochondria are seen in kidney cells and in brain cells. People with 3q29 deletion syndrome also tend to be smaller than normal, possibly because of altered fat metabolism.
The research was supported by the Brain & Behavior Research Foundation and the National Institute of Mental Health (F32MH124273, R56MH116994, R01MH110701, R01MH118534), and the Georgia Clinical & Translational Science Alliance, I3 (Think, Innovate) from Innovate and Innovate I- Emory University School of Medicine and a gift from the Woodruff Fund.
#Insights #schizophrenia #Genetic #risk #damages #mitochondria #Emory #University #Atlanta