× nearby
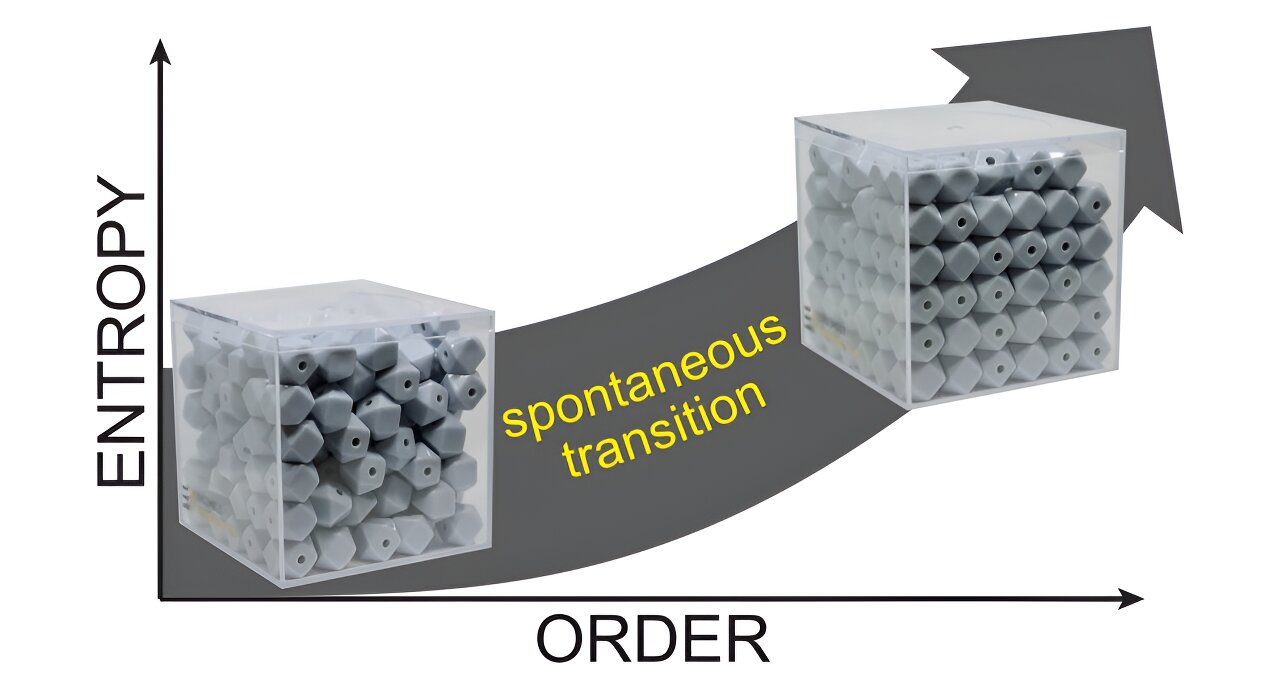
This hands-on model raises the known conditions of a simple system of complex particles to show how entropy is related to the number of accessible microstates by considering the degree of freedom available to each particle. Credit: T. Ryan Rogers
Although a cornerstone of thermodynamics, entropy remains one of the most troubling concepts to teach emerging physicists in the classroom. As a result, many people oversimplify the concept as a measure of disturbance in the universe, ignoring its fundamental nature.
In a Teacher of Physics, researcher T. Ryan Rogers created a hand-held model to demonstrate the concept of entropy to students. Using everyday tools, Rogers’ method allows students to approach the topic with a new intuition—one that takes some aim at the confusion between entropy and disorder.
“It’s a huge roadblock,” Rogers said. “The good news is that we’ve found that it’s something you can easily fix right away. The bad news is that this misunderstanding is taught right away.”
While many classes prefer imperfection, the standard shorthand for calling entropy “disorder,” is defined mathematically as the number of ways energy can be distributed in a system. Such an explanation simply requires students to understand how particles store energy, known formally as “degrees of freedom.”
To deal with the problem, Rogers developed a model in which small objects such as dice and buttons are poured into a box, replicating a simple thermodynamic process. Some particles in a dense box are packed in place, meaning they have fewer degrees of freedom, leading to a lower system entropy.
As the students shake the box, they bring energy to the system, releasing the locked pieces. This increases the total number of power distribution paths within the box.
“You’re basically zooming in on entropy so the students say, ‘Aha! There’s where I saw entropy increase,'” Rogers said.
As the pupils move further, the particles settle into a configuration that equally divides the energy between them. The catch: at this level of high entropy, the particles fall in an ordered arrangement.
“Even though it looks more ordered, there’s higher entropy,” Rogers said.
All students who participated in the study were able to reason about the correct definition of entropy after the test.
Next, Rogers plans to expand the reach of the model by starting a conversation about entropy with other teachers and creating a comprehensive work guide on how to use the kits for kindergarten through college. She hopes her work inspires others to make a difference in their classrooms, even if it’s DIY.
“Rais and Cheez-It crackers work really well, too,” Rogers said.
The article, “Hands-on Models for Researching Entropy and Disorder in the Classroom,” was written by T. Ryan Rogers and published on. Teacher of Physics.
More information:
T. Ryan Rogers, A Hands-on Model for Investigating Entropy and Disorder in the Classroom, Teacher of Physics (2023). DOI: 10.1119/5.0089761
#Capturing #entropy #Teachers #students #investigate #thermodynamics #handson #models