& bullet; Physics 16, 155
Observing the coalescence of air bubbles in water explains why dissolved salt slows down this process and leads to foaming.
Michael Sapryhin/stock.adobe.com
Air bubbles blown into clean water can easily collide. But bubbles coalesce more slowly in seawater or other liquids containing dissolved impurities, which is why these liquids often produce persistent foam. Now a team of engineers believe they have discovered the real cause of this difference—the subtle energy created by electrolytes, the mobile ions that are created when things dissolve in liquid. [1]. In a collision between two bubbles, this force greatly reduces the rate at which the liquid separating the bubbles can flow. This understanding, the researchers say, explains why foams form easily in salty seawater and could be useful in many industries.
Solutions with a high electrolyte concentration often produce continuous bubbles, so researchers have suspected for decades that it dissolves the electrolyte in some way by slow bubble formation. The result has remained mysterious, however, and many theories have even suggested that the electrolyte should accelerate the fusion of the bubbles, says mechanical engineer Bo Liu of the University of Alberta in Canada.
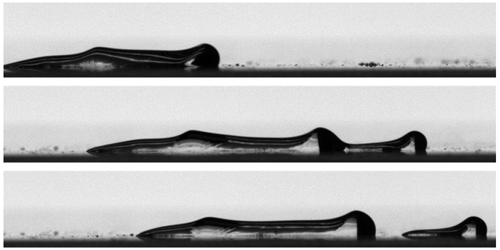
So, Liu and his colleagues undertook a series of experiments to test more precisely how the presence of electrolyte affects the formation of bubbles. They submerge the end of a glass capillary below a liquid surface and create an air bubble at the tip. They then force the bubble to descend at a speed of 3 mm/s until it connects with the bottom bubble attached to the silica surface. Using interferometry, the team can measure the thickness of the liquid film that separates the bubbles with nanometer precision and monitor this thickness as it shrinks to zero.
In pure water, the bubbles behaved like solid spheres, coming together without changing shape and joining together. However, bubbles in various electrolyte solutions are involved in a different, two-stage aggregation process. At first, the bubble surfaces are close, like in pure water. But when the separation went down to 40 nanometers (nm), the “leading edge” of the approaching surfaces flattened out as if there was a repulsive force. This flatness delayed bubble coalescence by 2 to 14 milliseconds, according to experiments with a selection of electrolytes and bubbles of different sizes.
These experiments, says Liu, are the first to clearly show that the presence of an electrolyte slows down bubble coalescence at the final stage, when the liquid film between the bubbles is very thin. But explaining the effect theoretically proved difficult. Working with various colleagues, team member Rogerio Manica, also of the University of Alberta, has spent years studying bubble coalescence and, in particular, the physics that influence how the thin liquid film between two approaching bubbles can separate. However, he says, “we didn’t have anything in hand that could explain the details of the observed test.”
In studying the results of other experiments, however, Liu, Manica, and their colleagues noticed a large difference in surface tension values for many electrolyte solutions compared to pure water. This observation, says Manica, encouraged them to develop a detailed mathematical model of electrolyte transport in the thin film between the contact bubbles. Using fluid dynamics equations, they were able to explain how the flow of electrolytes should influence the surface tension of the film.
The researchers found that when the thickness of the film decreases to 30-50 nm, there is a difference in electrolyte concentration between the film and the rest of the liquid. This difference produces less surface tension and a corresponding force that slows the flow of liquid out of the film.
In the models of the transport equations, the researchers found, this effect reduces the water transfer film enough to delay the breaking of the film – and the formation of the last bubble – in exact agreement with the experiment. Liu says: “In short, the electrolyte significantly delays the coalescence of bubbles by extending the life of the thin liquid film.”
This situation explains why whitecaps thrive in saltwater oceans, which contain a lot of electrolytes, but are not common in freshwater rivers and lakes, Liu said. This new understanding, he suggests, may find other industrial applications in the future, for example, in the electrochemical splitting of water molecules to produce hydrogen. In this process, the ways in which bubbles form and aggregate in the solution have a fundamental effect on the energy used and the efficiency of the product.
Building materials scientist Adrien Bussonière of the French National Center for Scientific Research (CNRS) in Paris says: “This is a very good job. He notes that the method identified by the researchers includes results on both the nanoscale, where each ion interacts with a thin film, and the very large scale where fluid flow phenomena operate. “This mechanism appears to be common to various salts and solves many unexplained electrolyte experiments.”
– Mark Buchanan
Mark Buchanan is a freelance science writer who divides his time between Abergavenny, UK, and Notre Dame de Courson, France.
References
- B. Liu continuous.“Nanoscale transport during liquid film reduction inhibits bubble coalescing behavior in electrolyte solutions,” Phys. Pastor Lett. 131104003 (2023).
Areas of Study
#Sea #Water #Foamy